▎ACHIEVEMENTS
NTU Reveals the Function of ER Stress-Induced Golgi Retrograde Transports
Share:
Endoplasmic reticulum (ER) and Golgi are the two major organelles in cells that support protein folding, modification, and subsequent transport. If the protein folding capacity of ER is overwhelmed or damaged, it induces the unfolded protein response (UPR), which acts to reduce ER stress. Research shows that ER stress causes cell death, and severe ER stress leads to diabetes, cancer, alteration in immune functions, and neurodegeneration, such as Alzheimer's disease, Parkinson's disease, and Huntington's disease. Unveiling the biology of ER stress and UPR benefits the development of putative clinical therapy strategies.
A research team led by Professor Fang-Jen Lee recently published their findings in Cell Reports. They had already discovered that ER stress activates small GTPase Arl1 at the Golgi and leads to Golgi recruitment of Arl1 effector golgin protein Imh1; however, the mechanism and function of the ER stress-stimulated Arl1-Imh1 pathway remained a mystery. To unravel the role of Golgi in the responses to ER stress, the team once again embarked on a mission to investigate the effects of UPR on the ER and the Golgi.
The team used a single-cell budding yeast (Saccharomyces cerevisiae) as a model organism for the research. In this study, they first discovered that ER stress would impair retrograde transport of the endosomal protein to the Golgi. The main retrograde transport to the Golgi depends on the GARP complex (Golgi-associated retrograde protein complex) and SNARE proteins. Under ER stress, the GARP complex is partially dysfunctional, which results in the failure of SNARE protein Snc1 and Tlg1 recycling transport to the Golgi. Therefore, the Golgi recruitment of Arl1 and Imh1 was enhanced to complement this defect. Furthermore, besides the Golgi localization, Imh1 was identified to be phosphorylated by mitogen-activated protein kinase (MAPK) Slt2/ERK2 to display its function in sustaining Snc1 and Tlg1 recycling transports in response to ER stress, which is independent of the ER stress master regulator Ire1 kinase.
This study offers remarkable insights into the functions of the Golgi under ER stress and reveals the communication between ER and the Golgi — laying a foundation for future studies on protein homeostasis and quality control in cells. According to Lee's team, their future goal is to focus on how the retrograde transport to the Golgi eases the burden of overwhelmed ER stress.
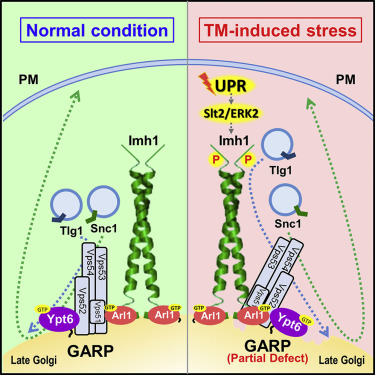
The team proved that the cooperative action of two different classes of tethers, golgin Imh1 and GARP complex, plays an essential role in recycling the transport of SNAREs under ER stress. The image shows how ER stress induces the MAP kinase Slt2/ERK2-dependent golgin Imh1 phosphorylation to suppress the defects of the GARP complex in SNAREs recycling transport.

