▎Achievements
Predicting Cancer Metastasis Through 3D Genome Organization
Share:
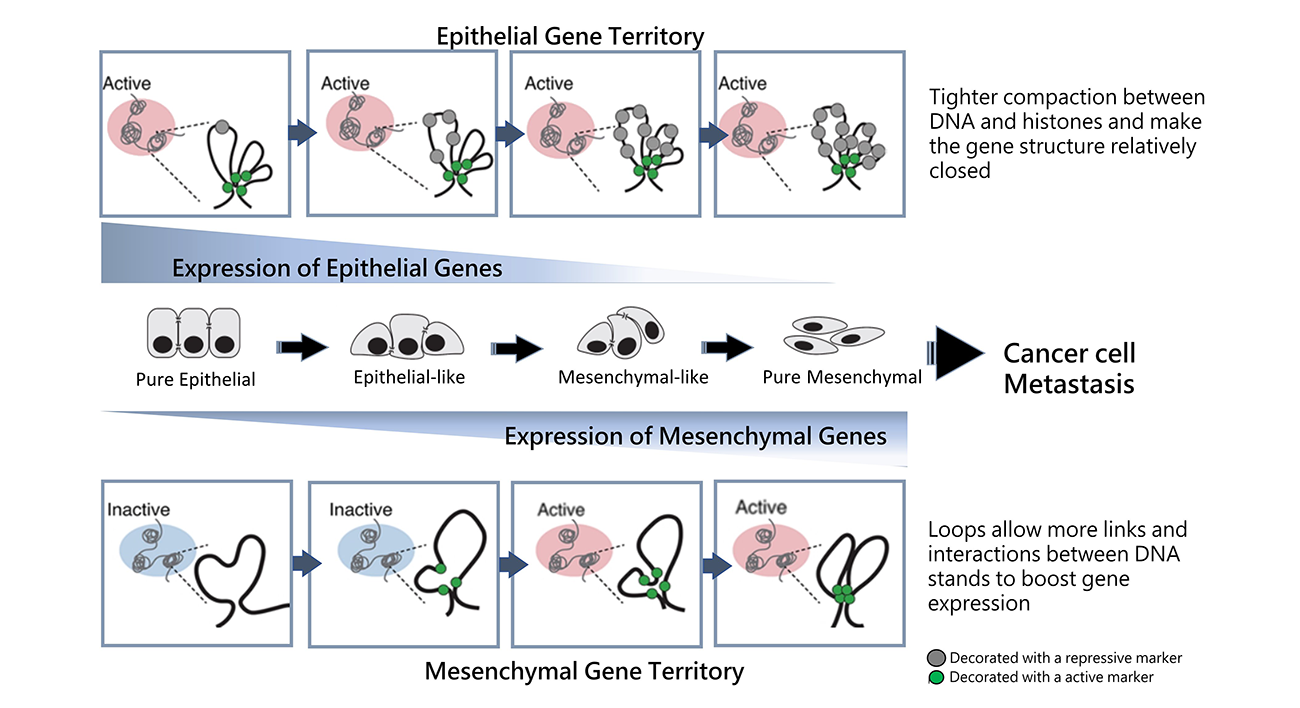
Illustration of 3D genome structure and epigenetic landscape changes in the epithelial and mesenchymal gene territory during different EMT states along the EMT spectrum.
Cancer metastasis is when cancer cells travel from the initial site to other tissues or organs of the body, forming new tumors. Metastasis is responsible for the great mortality rate in cancer patients and has always been one of the greatest challenges in oncology. The ability to predict metastasis risk would aid early intervention and timely treatment. Though many studies have tried to predict cancer metastasis based on the expression of specific genes, these methodologies are often either not holistic enough or inapplicable to a pan-cancer setting.
Recent research led by Professor Ruby Huang from NTU School of Medicine discovered evidence of architectural changes of the cancer cell genome at the three-dimensional (3D) level during the epithelial-mesenchymal transition (EMT) process. EMT is a fundamental step to embryonic development and wound healing, yet it is also leveraged by cancer cells to enable metastasis. The discovery was published in Genome Biology and sheds new light on cancer metastasis prediction and tumor progression.
With the support of researchers from the National University of Singapore, Prof. Huang’s team used high-dimensional capture of chromatin conformation (Hi-C) to discover how cancer cells have different states, including “pure epithelial” cells, "pure mesenchymal" cells, or “hybrid epithelial-mesenchymal” cells—forming an epithelial-mesenchymal transition spectrum (EMT Spectrum).
During the EMT process, the “pure epithelial” cells will reduce epithelial gene expressions and form slightly less compact “epithelial-like” cells. These “epithelial-like” cells will further promote the expression of mesenchymal genes to become “hybrid epithelial-mesenchymal” or “mesenchymal-like” cells. Eventually, the epithelial genes are completely suppressed to become “pure mesenchymal” cells. Huang discovered that during this process, the cancer must modify the cell’s genomic structure to facilitate DNA strand interactions.
With the single-cell Hi-C method, Huang not only observed the heterogeneity of cancer cells but also revealed that metastasis can be predicted through detecting 3D conformational changes of chromosomes 2 and 10. This major discovery unveils the complexity of EMT and opens the door to predicting cancer progression.

