▎Achievements
Mechanism of Fibronectin-Induced Small GTPase Stability and Promotes Cell Migration
Share:
Professor Fang-Jen Lee’s Research Reveals the Mechanism of Fibronectin-Induced Small GTPase Stability and Promotes Cell Migration.
Professor Fang-Jen Lee, Ph.D. student Ming-Chieh Lin, and the research team from the NTU Institute of Molecular Medicine recently published a paper that was featured in the Proceedings of the National Academy of Sciences, an authoritative international science journal, on July 20, 2022.
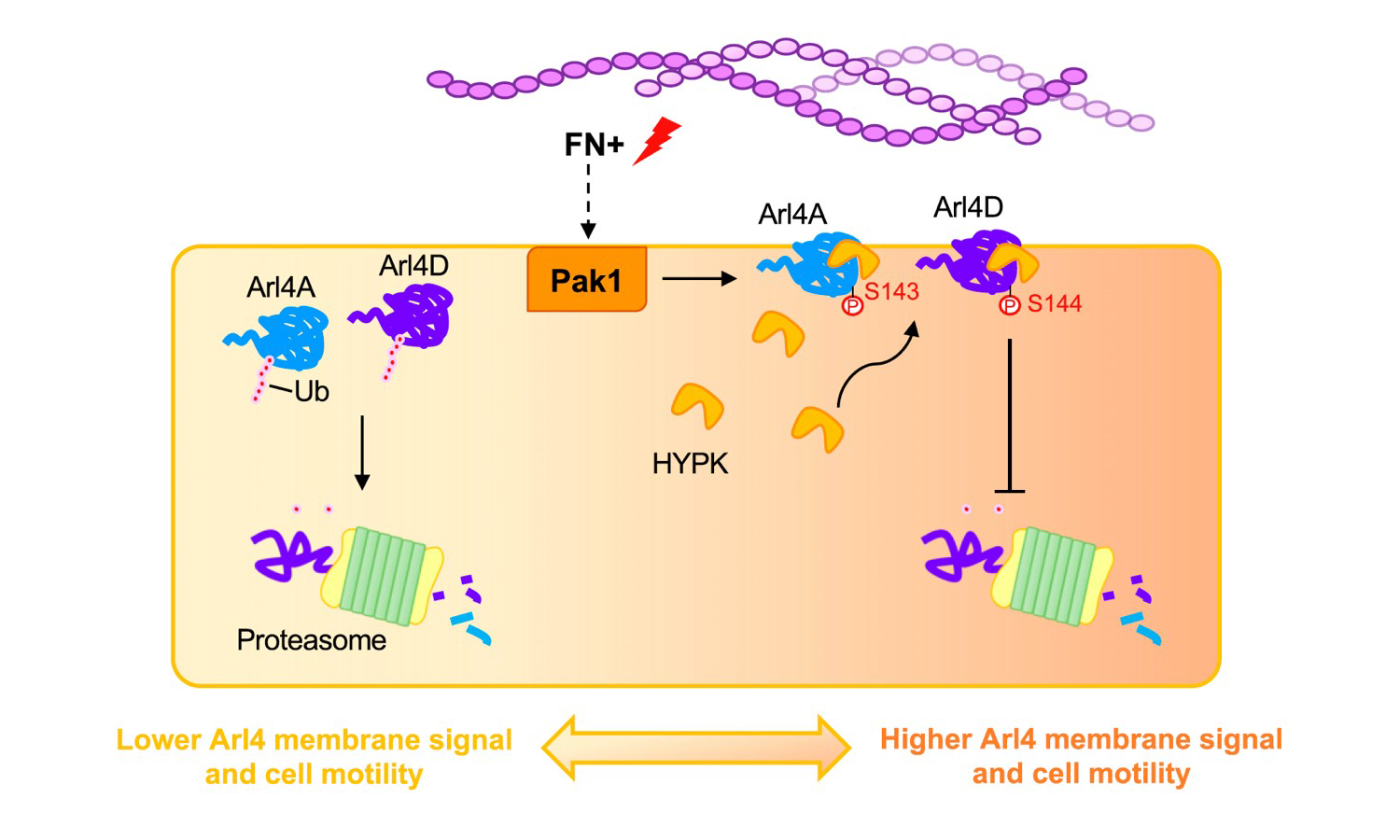
The role that GTPase Arl4A/D plays in cell migration has always been a mystery. Through relentless research, Lee and his team discovered for the first time that fibronectin can trigger small GTPase Arl4A/D proteins stability and enhance cell migration. This finding is a huge step towards understanding the molecular mechanism by which fibronectin guides cell migration or induces metastasis.
Cell migration is a fundamental process that involves the coordination and arrangement of molecules inside and outside the cell. This complex procedure is key to individual development and the evolution of a single cell to an adult organism. Fibronectin is an important extracellular matrix component, essential to the communication between intra and extracellular environment, guiding the adhesion of other extracellular matrices and the migration of cancer cells under pathological conditions.
Cells attached to fibronectin will induce intracellular signals, leading to elevation of Pak1 kinase activity which should be finely tuned in the cell migration processes. Numerous studies have shown that abnormal proliferation of Pak1 gene is highly oncogenic and associated with Parkinson's disease. Lee’s team has already made the discovery that feedback regulation between Arl4A and Pak1 activates Pak1 and promotes cell migration. By digging deeper into how extracellular signals regulate the operation of these molecules, we can better understand the complexity of cancer cell migration mechanisms.
Lee has long dedicated himself to elucidating the mechanism of Arl4 small GTPases signaling involved in cancer development. His latest study reveals that fibronectin induces Pak1-dependent phosphorylation of Arl4A/D, which enables the chaperone protein HYPK to bind small GTPases. This process results in the prevention of Arl4A/D proteasomal degradation and promotes their targeting the plasma membrane for cell motility. Now, thanks to this groundbreaking discovery, there will be a new research direction for the control of Pak1 kinase and small GTPase-induced cancer cell migration and related clinical diseases.
This research was supported by the National Health Research Institutes and the Ministry of Education.

